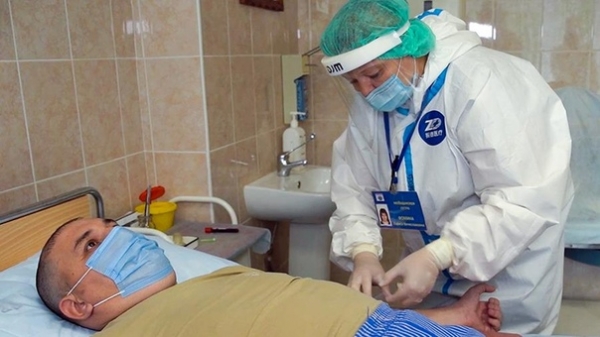
Vaccine for the prevention of COVID-19, developed by the American company Moderna, caused a strong immune response in all participants in the first stage of human trials should be published in the journal New England Journal of Medicine.
The first stage of testing was completed in late may. The study involved 45 people, divided into three groups. They all received two doses of vaccine, 25, 100 or 250 micrograms. As a result, the company claims, they’ve all developed antibodies, and in patients from the group received high dose, the level was several times higher than the ill COVID-19.
According to the representative of Moderna Dr. Tala Sachs, these data suggest that the vaccine triggers the immune response at all dose levels, and 100 micrograms would be the optimal dose in the third stage of the research.
“We look forward to studies of the third phase of mRNA-1273 this month to demonstrate the ability of our vaccine to significantly reduce the risk of disease COVID-19″ —said Sachs.
The company also emphasizes that tests have confirmed the safety of the vaccine.
To large-scale clinical trials, Moderna is expected to start on July 27. In them will take part about 30 thousand volunteers, each of whom will receive a dose of 0.1 milligram. 29 days of repeat injection. Some participants will be vaccinated with the drug-placebo.
The development of Moderna supports the US Federal government, which has allocated almost $ 500 million. However, as reported last week, Moderna clashed with government scientists, has not sent on time test reports and rejected the advice of experts on their implementation. As a result, this tension has led to delayed tests for more than two weeks.








More Stories
Color wheel online
The Ministry of health has promised to disclose information about the vaccine trials from COVID-19
Called “atypical” symptoms of coronavirus